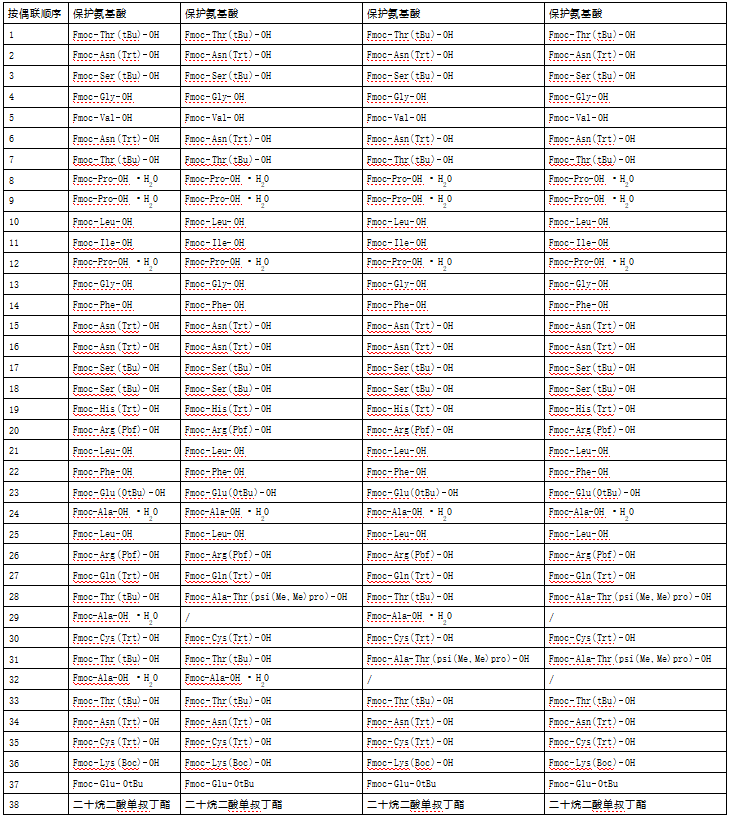
PEGylation Of Calcitonin
Calcitonin (CT) is a 32 peptide hormone secreted by parafollicular cells (C cells) in the thyroid gland. It was first discovered and demonstrated by Hirsh to regulate calcium phosphorus metabolism. At present, calcitonin has shown good efficacy in the treatment of osteoporosis, Paget's disease, hypercalcemia, hyperparathyroidism, osteoarthritis, and bone healing. However, during the use of calcitonin, it has also been found that the drug has common drawbacks as protein and peptide drugs, such as immunogenicity and short half-life. There have been clinical reports that calcitonin can cause allergic reactions. In addition, due to the half-life of calcitonin in the human body being 70-90 minutes and its rapid metabolism, it needs to be administered daily or every other day, which also brings difficulties and pain to patients in treatment.
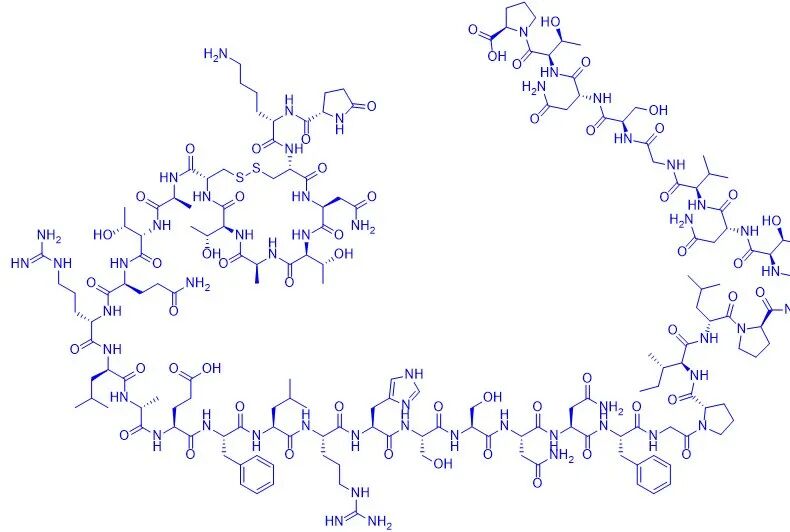
In this study, a polyethylene glycol-modified calcitonin was provided, characterized by a long chain of polyethylene glycol derivatives attached to the amino group of calcitonin by covalent bonds, and the group directly linked to the amino group of calcitonin was methylene or carbonyl. Using salmon calcitonin, the amino acid composition sequence of salmon calcitonin is:
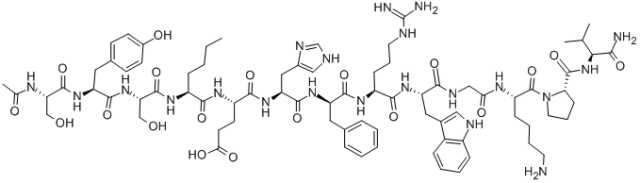
H-Cys-Ser-Asn-Leu-Ser-Thr-Cys-Val-Leu-Gly-【Lys】-Leu-Ser-Gln-Glu-Leu-His-【Lys】-Leu-Gln-Thr-Tyr-Pro-Arg-Thr-Asn-Thr-Gly-Ser-Gly-Thr-【Pro】-NH2( Two Cys can form disulfide bonds; The molecular formula is C145H240N44O48S2; The molecular weight is 3432.
Example 1 mPEG-SPA-5000 modifies the synthesis of salmon calcitonin
Selection of reaction temperature:
Take 2ml of 1.0mg/ml salmon calcitonin solution, add 2ml of phosphate buffer solution to make the pH value of the solution 7.0, then add 15mg of mPEG-SPA-5000 solid, dissolve, mix well, take 0.8ml each and place it in 4 stoppered test tubes, and then place them at 4 ℃, 10 ℃, 25 ℃, and 37 ℃ for 30 minutes. Add 5mg of glycine to terminate the reaction. Compare the modification rate of single polyethylene glycol modified salmon calcitonin (a salmon calcitonin molecule with one polyethylene glycol chain attached, hereinafter referred to as polyethylene glycol modified salmon calcitonin) and determine the modification conditions.
The results showed that polyethylene glycol-modified salmon calcitonin could be obtained at these temperatures, and the modification rate was the highest at 25°C.
Selection of reaction time:
Take 2ml of 1.0mg/ml salmon calcitonin solution, add 2ml of phosphate buffer to make the pH value of the solution 7.0, then add 15mg of mPEG-SPA-5000 solid, dissolve, mix well, take 0.7ml of each and put it in 4 corked test tubes, and then react at 25°C for 5, 15, 30, 60 and 120 min, then add 5mg glycine to stop the reaction. Compare the modification rate and determine the modification conditions.
The results showed that pegylated salmon calcitonin could be obtained under these conditions, and the modification rate did not increase significantly after 30 minutes.
Molar ratio of mPEG-SPA-5000 to salmon calcitonin:
Take 8ml of 1.0mg/ml salmon calcitonin solution, add 8ml of phosphate buffer to make the pH value of the solution 7.0, take 3.0ml of each and put it in 5 corked test tubes, and then add 2.2, 4.4, 6.6, 11.0 and 22.0mg mPEG-SPA-5000 (equivalent to the molar ratio of salmon calcitonin and mPEG-SPA-5000 is 1∶1, 1∶2, 1∶3, 1∶5, 1∶10, respectively), dissolve, mix, and mix. The reaction is terminated after 30 minutes. Compare the modification rate and determine the modification conditions. The results showed that the polyethylene glycol-modified salmon calcitonin could be obtained under these conditions, and the modification rate reached the highest when the molar ratio of mPEG-SPA-5000 and salmon calcitonin was 3.
Example 2 Isolation, purification and identification of polyethylene glycol-modified salmon calcitonin
Take 1.0mg/ml of salmon calcitonin solution, add 10ml of phosphate buffer to make the pH value of the solution 7.0, then add 44mg of mPEG-SPA-5000 solid, dissolve, mix well, react at 25°C for 30min, and add 25mg glycine to stop the reaction. The above reaction solution was taken and replaced with an ultrafiltration membrane with an intercepted molecular weight of 1000, and the acetate-sodium acetate buffer with a pH of 4.0 was replaced and concentrated to 5ml, and separated on the column. The chromatographic conditions are as follows:
Chromatography medium: SOURCE30S
Column volume: 5ml
Flow rate: 3.0ml/m
in Column equilibration: Equilibrate 5 times the column volume with 0.01mol/L, pH 4.0 sodium acetate (starting buffer)
Loading volume: 5ml
Elution: The unadsorbed part is eluted with a starting buffer of 3 times the column volume, and then a gradient is used to elute the starting buffer, 1.0mol/LNaCl buffer, and the percentage of 1.0mol/LNaCl buffer volume is from 0~100%, and the column volume is eluted 20 times the column volume. Collection: 3.0ml/tube was followed up with RP-HPLC, combined with polyethylene glycol-modified salmon calcitonin, and purified by reversed-phase column chromatography. The chromatographic conditions for reversed-phase column chromatography are as follows: Chromatography medium: Reversed-phase silica gel (C18, 40 μm)
Column volume: 10ml
Flow rate: 3.0ml/m
in Column equilibration: 10 times the column volume equilibrated with 0.5%
acetic acid solution: 10ml
Elution: The unadsorbed part is eluted with 0.5% acetic acid solution with 5 times the column volume, and then the gradient solution is composed of 0.5% acetic acid solution and 0.5% acetate ethanol solution, and 0.5% acetate ethanol solution is used to elute 30 times the column volume. Collection: 3.0ml/tube was followed up with RP-HPLC and combined with pegylated salmon calcitonin.
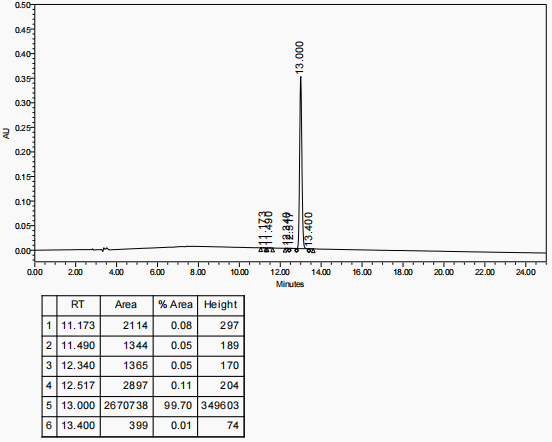
RP-HPLC analysis showed that the purity of the obtained polyethylene glycol-modified salmon calcitonin was more than 98%, as shown in Figure 1, which had a high purity.
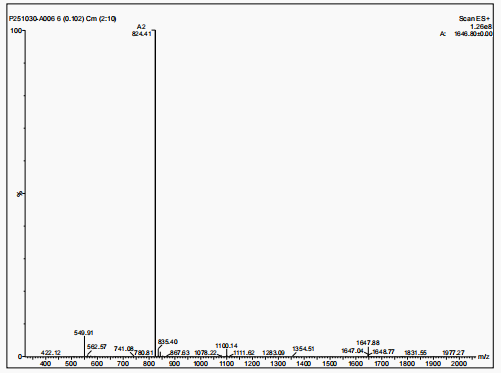
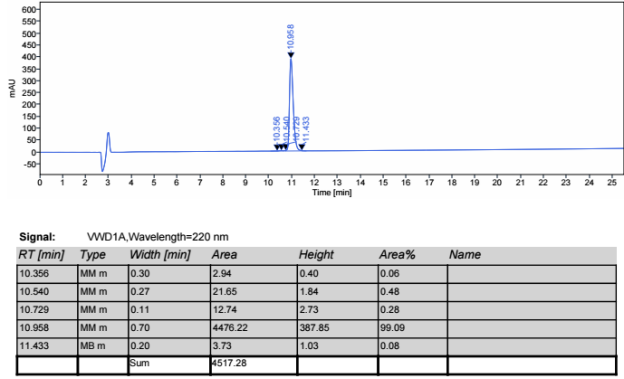
The molecular weight of polyethylene glycol-modified salmon calcitonin was measured to be 8187.31 by MALDI-TOF-MS, as shown in Figure 2.
The molecular weight of salmon calcitonin is 3432, the molecular weight difference between the two is about 5000, and there is a series of peaks near 8187.31 (M+1 peak), which has the typical structural characteristics of polyethylene glycol, which confirms that the polyethylene glycol-modified salmon calcitonin obtained in embodiment 2 is a single modification product.
Example 3 Comparison of salmon calcitonin and polyethylene glycol-modified salmon calcitonin (prepared by the method of embodiment 2) to reduce blood calcimon in rats
Referring to the potency determination method of calcitonin in Appendix XIIO of Part II of the Chinese Pharmacopoeia in 2005, 90 Wistar female rats weighing 200±15g were selected and fasted for 16 hours before the test, freely drank distilled water, and were randomly divided into 3 groups, with 30 blank, salmon calcitonin and polyethylene glycol-modified salmon calcitonin groups. The dose of salmon calcitonin and pegylated glycol-modified salmon calcitonin was 0.05 μg/kg (calculated by the amount of salmon calcitonin in pegylated glycol-modified salmon calcitonin), and the volume was 0.4 ml/100 g. Before administration and 1, 2, 4, 8, and 12 hours after administration, 5 animals were taken from each group, blood was collected from the ocular venous plexus, and the blood calcium value in the samples was determined by o-cresol phthalein complex. The average blood calcium value of the blank group at each time point is subtracted from the average blood calcium value of the administration group, and the difference between the two is used to compare the average blood calcium value of the blank group with the average blood calcium value of the upper blank group, that is, the blood calcium level can be reduced. The results showed that the polyethylene glycol-modified salmon calcitonin not only did not decrease, but also increased significantly, and the level of blood calcium reduced by 1 times at the same dose was also significantly extended, from 4 hours to 8 hours before modification.
Example 4 Study of the immunogenicity of polyethylene glycol-modified salmon calcitonin (prepared by the method of embodiment 2) on experimental animals
Rabbits were used as experimental animals to prepare antiserum, and formaldehyde-treated salmon calcitonin and polyethylene glycol-modified salmon calcitonin were used as antigens, at a dose of 0.5mg/kg/time, once a week, for a total of 5 times. Salmon calcitonin and pegylated salmon calcitonin were used as antigens, and their respective antiserum titers were determined by two-way immunodiffusion assay, and the results showed that the antiserum titers of salmon calcitonin group were 1∶32. The antiserum titer of the salmon calcitonin group modified by polyethylene glycol could not be measured. Salmon calcitonin and polyethylene glycol-modified salmon calcitonin were used as antigens, and then the antiserum of the salmon calcitonin group was used as the primary antibody, and then horseradish peroxidase (HRP)-labeled sheep anti-rabbit IgG was used as the secondary antibody, and their respective immunogenicity was determined by enzyme-linked immunosorbent assay (ELISA), and the results were as follows: salmon calcitonin group was positive, and pegylated salmon calcitonin group was negative. The above results show that compared with salmon calcitonin, the immunogenicity of pegylated salmon calcitonin modified by polyethylene glycol is significantly reduced, which is more conducive to its use as a therapeutic drug.
Example 5 mPEG-SPA-2000, mPEG-SPA-10000, mPEG-SPA-20000, mPEG-SPA-30000 mPEG-SPA-60000 replace mPEG-SPA-50000 in embodiment 1, and use the methods of embodiments 1 and 2 to obtain polyethylene glycol-modified salmon calcitonin, and perform experiments such as embodiments 3 and 4 to obtain similar results.
Example 6 mPEG-SBA-2000, mPEG-SBA-5000, mPEG-SBA-10000, mPEG-SBA-20000, mPEG-SBA-30000 or mPEG-SBA-60000 instead of mPEG-SPA-5000 in embodiment 1, using the methods of embodiments 1 and 2 to obtain polyethylene glycol-modified salmon calcitonin, and conducting experiments such as embodiments 3 and 4 to obtain similar results.
Example 7 Selection of reaction temperature of salmon calcitonin modified with methoxy polyethylene glycol propionaldehyde 5000 (mPEG-ALD-5000): take 2ml of 1.0mg/ml salmon calcitonin solution, add 2ml of phosphate buffer to make the pH value of the solution 5.0, then add 30mg of mPEG-ALD-5000 solid, dissolve, mix well, and then add 0.029ml of 1mol/L sodium cyanobohydride, Take 0.8ml of each and place it in 4 plugged test tubes, then put it at 4°C, 25°C, 37°C and 50°C for 16h, and then add 5mg glycine to stop the reaction. Compare the modification rate and determine the modification conditions. The results showed that polyethylene glycol-modified salmon calcitonin could be obtained at these temperatures, and the modification rate was the highest at 37°C.
Selection of reaction time: take 2ml of 1.0mg/ml salmon calcitonin solution, add 2ml of phosphate buffer to make the pH value of the solution 5.0, then add 30mg of mPEG-ALD-5000 solid, dissolve, mix well, add 0.029ml of 1mol/L sodium cyanoboron hydride, take 0.7ml of each and put it in 5 corked test tubes, and then react at 37°C for 0.5, 1.0, 8.0, 16.0, 24.0h, and then add 5mg glycine to terminate the reaction. Compare the modification rate and determine the modification conditions. The results showed that salmon calcitonin modified by polyethylene glycol could be obtained under these conditions, and the modification rate did not increase significantly after 16 hours.
Selection of molar ratio of mPEG-ALD-5000 to salmon calcitonin: take 8ml of 1.0mg/ml salmon calcitonin solution, add 8ml of phosphate buffer to make the pH value of the solution 5.0, take 3ml of each and put it in 5 corked test tubes, and then add 2.2, 6.6, 11.0, 22.0 and 33.0mg of mPEG-ALD-5000 (equivalent to the molar ratio of calcitonin to mPEG-ALD-5000 in 1∶1~1∶15), Dissolve, mix well, then add 0.022ml of 1mol/L sodium cyanobohydride for 16.0h, and then add 5mg glycine to stop the reaction. Compare the modification rate and determine the modification conditions. The results showed that the modification rate of salmon calcitonin modified with pegylated glycol was the highest when the molar ratio of mPEG-ALD-5000 to calcitonin was 10, and the modification rate of mPEG-ALD-5000 was not significantly increased.
Example 8 Separation, purification and identification of polyethylene glycol modified salmon calcitonin (aldehyde modification) Take 10ml of salmon calcitonin solution at 1.0mg/ml, add 10ml of phosphate buffer to make the pH value of the solution 5.0, then add 150mg of mPEG-ALD-5000 solid, dissolve, mix well, add 0.145ml of 1mol/L sodium cyanoborohydride, react at 37°C for 16h, and then add 50mg of glycine solid to stop the reaction. Then, according to the method in embodiment 2, the separation, purification and identification of polyethylene glycol-modified calcitonin are carried out. RP-HPLC analysis showed that the purity of the obtained polyethylene glycol-modified salmon calcitonin was more than 98%, which was relatively high. MALDI-TOF-MS analysis showed that its molecular weight was 8124.21, which was a monomodified polyethylene glycol-modified salmon calcitonin.
Example 9 Study on the characteristics of polyethylene glycol modified salmon calcitonin (aldehyde modification) The test and immunogenicity study of mPEG-ALD-5000 modified salmon calcitonin to reduce blood calcimon in rats were carried out according to the method in embodiment 3~4, and the results not only did not decrease the titer, but also significantly increased, the action time was significantly prolonged, and the immunogenicity was reduced.
Example 10 replacing mPEG-ALD-5000 in embodiment 7 with mPEG-ALD-2000, mPEG-ALD-10000, mPEG-ALD-20000, mPEG-ALD-30000 or mPEG-ALD-60000, using the methods of embodiments 7 and 8 to obtain polyethylene glycol-modified salmon calcitonin, and conducting experiments as in embodiment 9 to obtain similar results.
Example 11 mPEG-bALD-2000, mPEG-bALD-5000, mPEG-bALD-10000, mPEG-bALD-20000, mPEG-bALD-30000 or methoxy polyethylene glycol butyraldehyde 60000 (mPEG-ButyrALD-60000) are used instead of mPEG-ALD-5000 in embodiment 7, and polyethylene glycol modified salmon calcitonin is obtained by the methods of embodiments 7 and 8 , and conduct experiments such as the embodiment 9 to obtain similar results.
We are a professional researcher and manufacturer of pharmaceutical polymer materials, providing gram, kilogram, tonnGMP and GMP pharmaceutical PEG, PLA, PLGA, and provide complete quality research reports. We are committed to escorting the development of your medical devices and welcoming more patients.
Email address: haoran.tse@gmail.com
Tel: +8618575536586